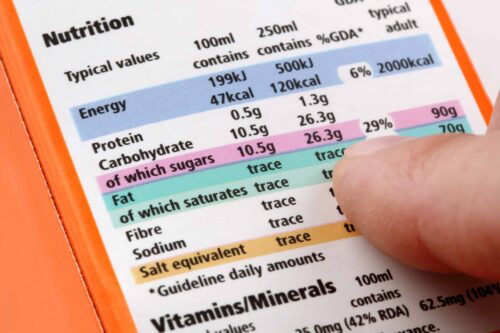
Quality of food supplements – Europe
FoodChain ID and NOW Foods Partner to Showcase Regulatory Leadership
How to create a compliant supplement label for Europe?
What are the requirements for selling supplements online in Europe?
Novel foods and food supplements: understanding the European legal framework
What are health claims?
Service details
Efficacy and safety literature review
Our experts support organizations through research, analysis and synthesis of data related to the efficacy/ physiological effect/safety of ingredients when taken orally, according to existing scientific literature published in peer-reviewed journals and aligned with regulatory requirements.
Plants and substances appendices (France)
- Constitution of characterization files on plant preparations (Appendix II)
- Establishment of safety records on non-traditional preparations of plants (Appendix III)
- Establishment of efficiency and safety records on substances (Appendix II)