FoodChain ID and NOW Foods Partner to Showcase Regulatory Leadership
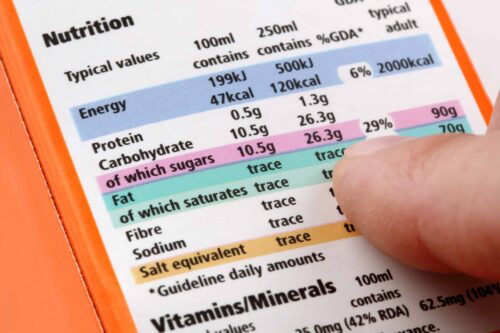
How to create a compliant supplement label for Europe?
What are the requirements for selling supplements online in Europe?
Novel foods and food supplements: understanding the European legal framework
What are health claims?
What are the Novel Foods ?
How to sell Dietary Supplements on Amazon
Service details
Formulation development according to :
- Appropriate regulatory conditions (regulatory status, specific countries, claims)
- Specific requirements (galenic form, quality, price)
- Health benefits and safety
Formulation
Research, analysis, selection and recommendation of ingredients according to their scientific substantiation and regulatory compliance.
Development of a theoretical formulation, taking into account appropriate efficacy and safety dosage of selected ingredients, potential additional ingredients more likely to be identified and recognized by consumers, other ingredients that may allow the use of health claims.
Galenic development
- Identification of suitable contract manufacturers depending on the galenic form.
Scientific substantiation
- Completion of a scientific file substantiating the efficacy and interest of the ingredients used for a targeted health application, with bibliographic reference.
Regulatory authorization
Validation of the authorization of the selected ingredients in accordance with specifications and selected dosages.
- Support for label compliance
- Notification registration through authorities