Regulatory, Scientific and Marketing Data to create compliant Supplements
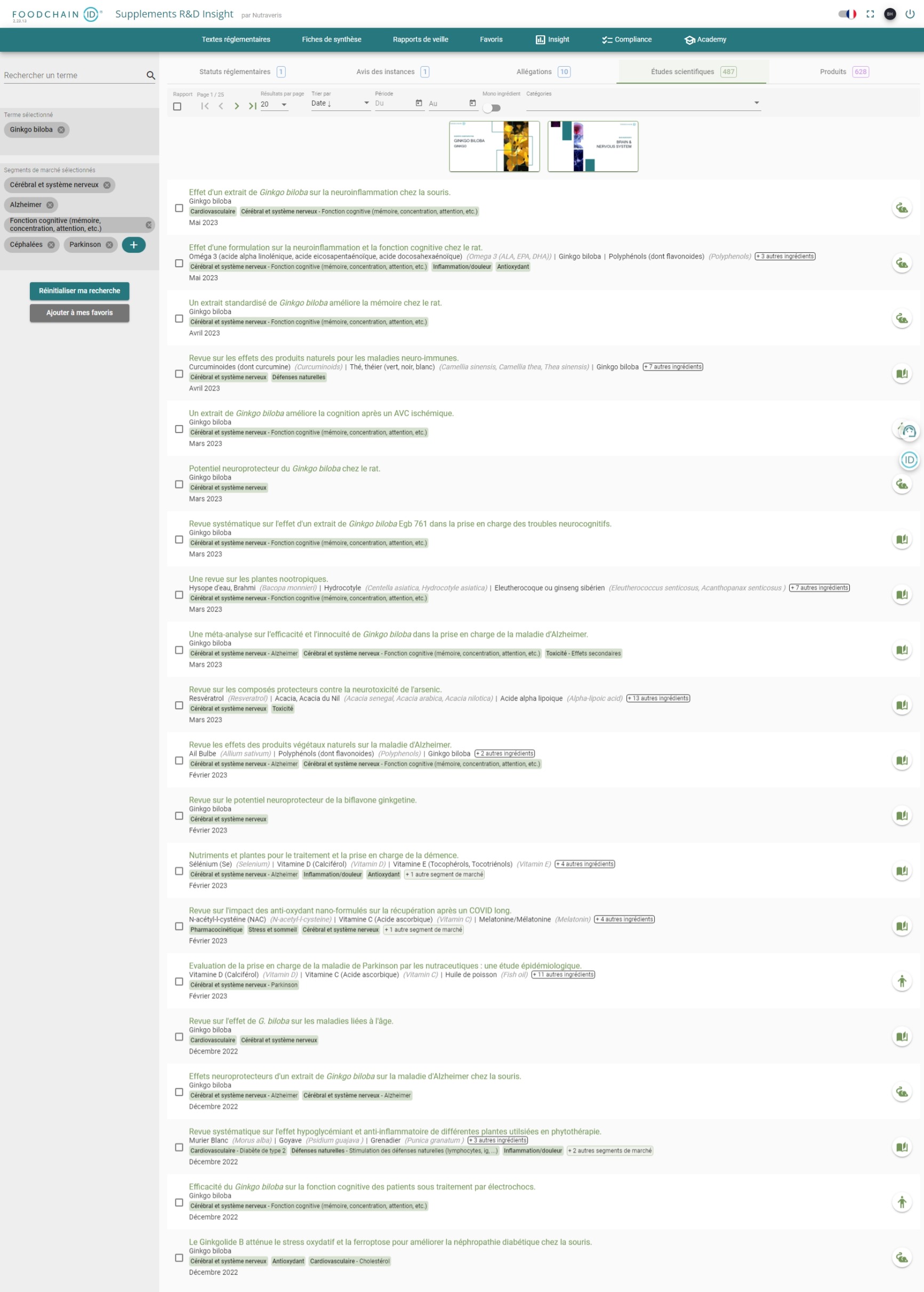
“Show me the scientific evidence of the effect of Ginkgo Biloba on brain function”
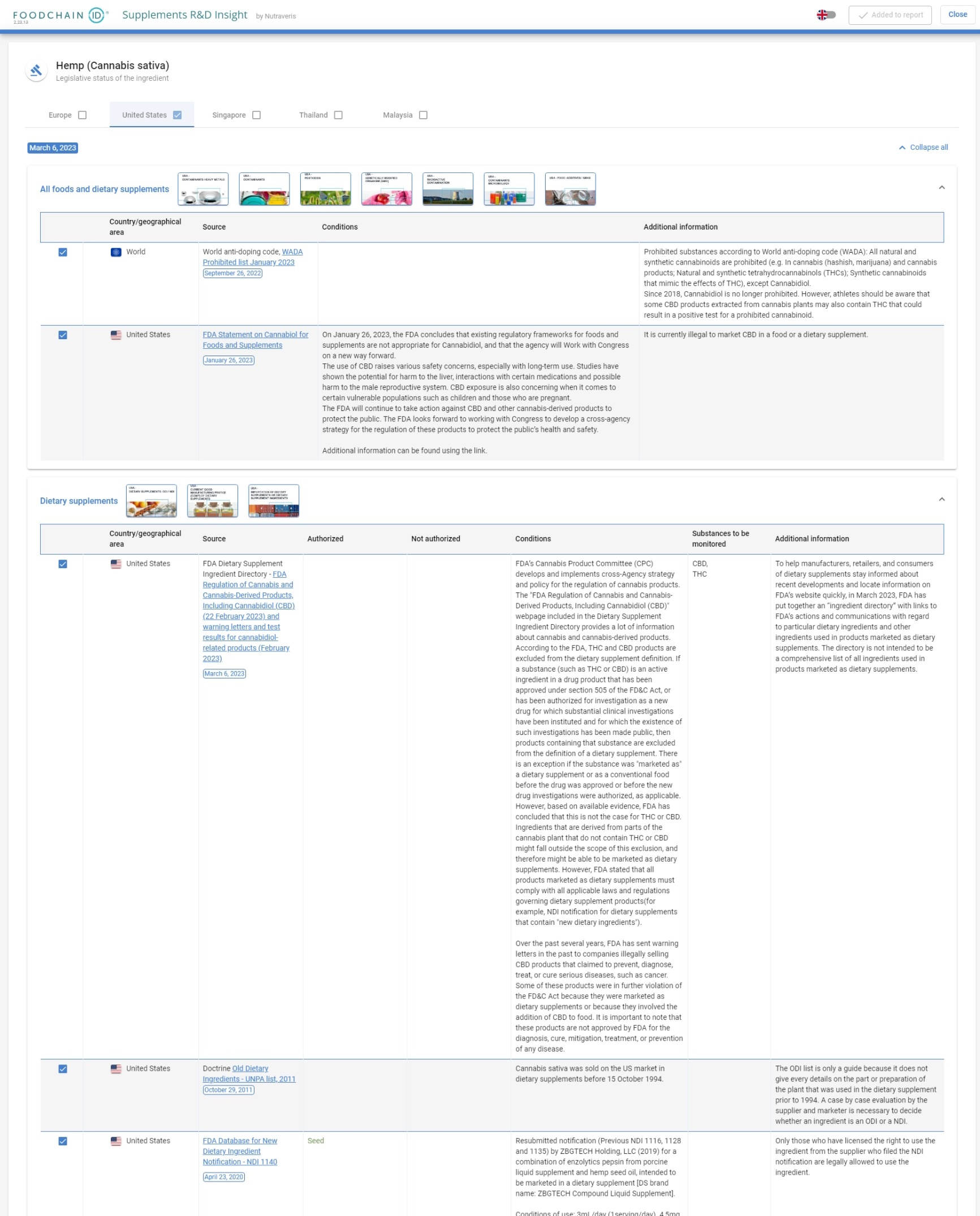
“What is the regulatory status of CBD in the US?
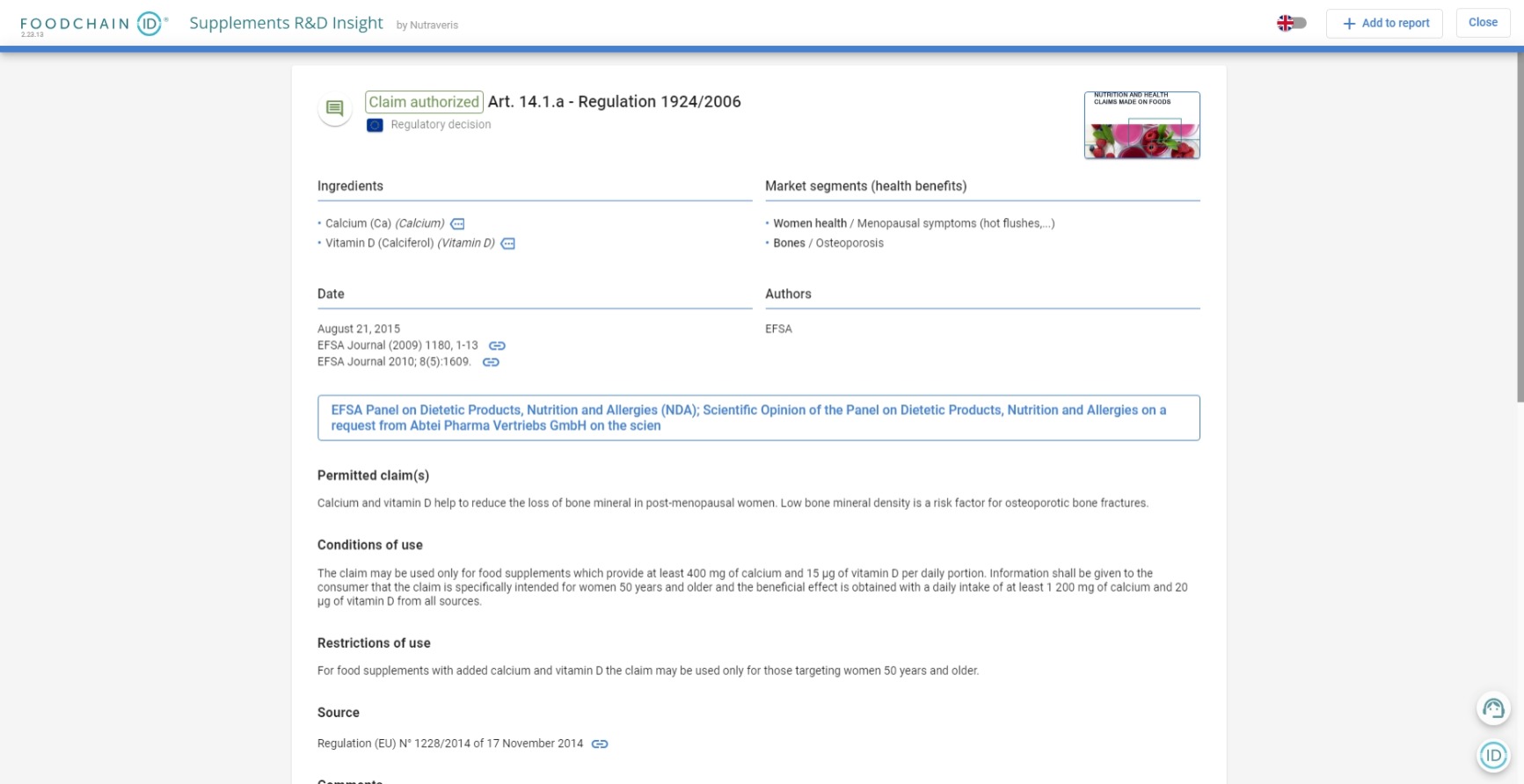
“Give me an example of an authorized claim for Vitamin C”

Regulatory Texts
Simplify regulatory compliance for your products
The tool provides easy access to up-to-date information on regulatory requirements for supplements, simplifying the compliance process for businesses when creating formulas and products.

US, EU and Asian Regulatory Data for 3,000 ingredients
Find the regulatory status of an ingredient and understand how it can be used (dosage, limits), what can be said about its health benefits (health claims) and how it can be sold.
Scientific Substantiation
Save time and resources
By providing comprehensive and current information on regulations and guidelines, Supplements R&D Insight saves businesses the time and resources traditionally spent researching and understanding regulatory requirements.

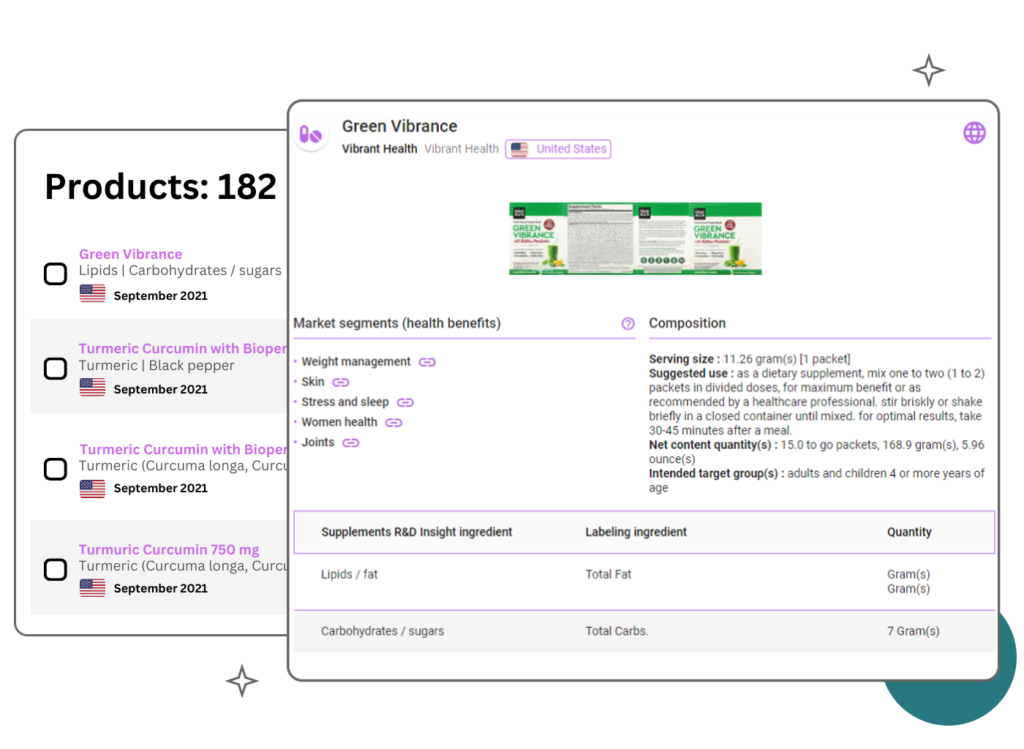
Products Benchmark
Enhance competitiveness
By enabling businesses to develop compliant, high-quality food supplements, the tool helps you stay competitive and meet the growing demand for safe, effective, and innovative products in the marketplace.

What's in the box?
Supplements R&D Insight
Ver 2.20
| Regulatory Status | 3,000 ingredients |
| Authority Opinions | 1,657 texts |
| Claims | 6,707 texts |
| Scientific Studies | 78,000 texts |
| Product Benchmarks | 185,000 sheets |
| EU Regulations | ✔ |
| US Regulations | ✔ |
| Asian Regulations | ASEAN, Thailand, Singapore, Malaysia |
| Daily Email Alerts | Automatic notifications of monitor ingredients, competitors, rules, health benefit with no effort |
| Custom Reports | Create your own reports by bookmarking results that can be shared in pdf, Excel, html formats and saved as an automatic alert |
| Monthly Reports | Published by our experts, our monthly report cover each regulatory zone, plus an additional scientific report |
| Mindmap | Merge all the links related to regulatory texts into a mindmap so you can get a deep understanding deeply of regulatory frameworks |
| Free Support | Free support hotline manned by our experts included |
| Summary Sheets | Scientific and regulatory summaries of important topics |

