Positive effect of N-acetylcysteine on remission maintenance in patients with ulcerative colitis
This double-blind randomized controlled clinical trial was conducted to assess the effect of N-acetylcysteine (NAC) on remission maintenance in patients with ulcerative colitis (UC).
To do so, 168 patients with UC were randomized to receive either 800 mg/d of NAC or placebo for 16 weeks. The primary efficacy of the treatment was remaining in remission. The secondary outcomes were the endoscopic relapse, serum level of hs-CRP, hemoglobin, and fecal calprotectin level.
Results showed that during 22 weeks follow up, 25 patients experienced relapses, six of them were in the NAC group and 19 of them were in the placebo group. There was a significant difference between the NAC and placebo groups regarding the relapse-free period (p = 0.007). Compared with the NAC group, significantly more patients in the placebo group had an endoscopic relapse (p < 0.001). At the end of the intervention period (16 weeks) and 6 weeks post-intervention, the mean fecal calprotectin, serum erythrocyte sedimentation rate, and hs-CRP levels were significantly lower in the NAC group compared with the placebo group (p < 0.05).
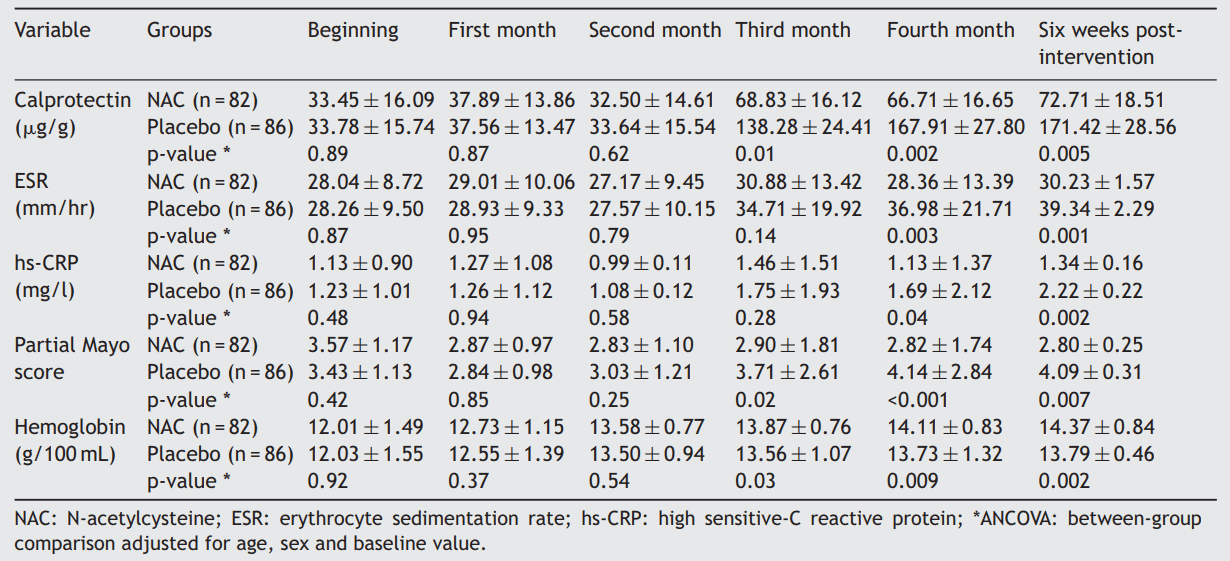
In conclusion, NAC has significantly more positive effect on the maintenance of remission compared with placebo in UC patients.
Masnadi Shirazi K, Sotoudeh S, Masnadi Shirazi A, Moaddab SY, Nourpanah Z, Nikniaz Z. Effect of N-acetylcysteine on remission maintenance in patients with ulcerative colitis: A randomized, double-blind controlled clinical trial. Clin Res Hepatol Gastroenterol. 2020 Oct 14:S2210-7401(20)30270-9.
