FoodChain ID and NOW Foods Partner to Showcase Regulatory Leadership
How to create a compliant supplement label for Europe?
What are the requirements for selling supplements online in Europe?
Novel foods and food supplements: understanding the European legal framework
What are health claims?
What are the Novel Foods ?
How to sell Dietary Supplements on Amazon
Service details
Our experts can help ensure the quality of your products and protect your brand by:
- Developing manufacturing subcontractor specifications (standards, rules, etc)
- Integrating European and other national quality standards (microbiological criteria, heavy metals, pesticides, solvent residues, GMOs, allergens and more)
- Evaluating procedures already in place (ingredient quality standards, controls, batch records, etc.)
Manufacturing subcontracting specifications review
Corresponding to the manufacturing framework of food supplements, and in particular indications of the regulatory texts applicable in Europe and/or a specific country, this covers delimitation of the subcontractor’s liability, constraints linked to the provision of the elements, etc.
Review of European and national quality standards in place
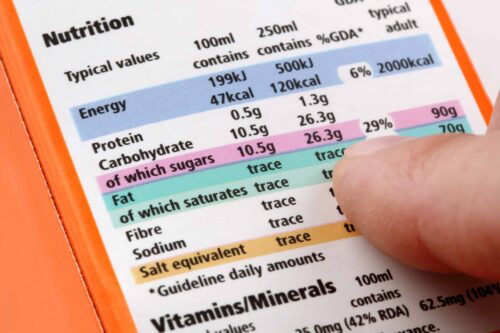
Summary of the requirements and quality standards applicable for the marketing of food supplements according to European regulations (microbiology, heavy metals, pesticides, solvent residues, GMOs, BSE, allergens, PAHs, dioxins, mycotoxins, irradiation etc.).
Evaluation of product ingredient quality according to European standards
Study of the technical documents of the ingredients of a finished product provided by the manufacturers (technical data sheet, specification sheets, safety data, certificates of analysis, manufacturing process chart etc.). Analysis of the quality criteria for each ingredient and comparison with current standards to determine compliance with regulatory requirements.
Quality control
Following receipt of samples and batch files after each production, assessment of compliance with specifications: qualitative and quantitative formula, criteria and quality controls. Keeping and making available files in the event of control by administrations.
Constitution of the technical file of the food supplement
Identification of essential information to characterize the product and describe its qualities (product identification, development, manufacturing, control of ingredients, control of the finished product, stability, storage and transport).